Amides May Be Prepared by Which of the Following Methods
An amide can be produced in a variety of ways. Both the reaction of an acid chloride with an amine and the reaction of an acid anhydride with ammonia are correct.

Amides Preparation And Reactions Summary Chemistry Steps
The first step is the nucleophiic addition of the carbonyl group to form an imine.

. An NNdisubstituted amide is prepared by reacting an acid halide with a secondary amine. The reaction of ammonia with an alkyl halide leads to the formation of a primary amine. Which block requires the anesthetic agent be injected into the muscle bundle behind the eye.
Phenyl isocyanides are prepared from which of the following reactions Related. Amines are prepared by the following methods. 1 From the reaction of an acyl halide with a 1 or 2 amine 2 the reaction of a carboxylic acid with an amine usually requires heating and 3 the reaction of an acid anhydride commonly acetic anhydride with an amine.
Amides may be prepared by which of the following methods. Now up your study game with Learn mode. The second step is the reduction of the imine to an amine using an reducing agent.
An Nsubstituted amide is prepared by reacting an acid halide with a primary amine. The reaction takes place in two parts. You just studied 57 terms.
Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. A method for preparing a benzoic acid amide derivative of Chemical Formula 1 which is expressed as Reaction Formula 1 and comprises a step of performing an amide coupling reaction of a benzyl amine compound of Chemical Formula 2 and an adamantanyl benzoic anhydride of Chemical Formula 3 under a base catalyst. Also nitro compounds can be reduced metals in an acidic environment to generate amines.
0 A the reaction of an acid chloride with an amine 0 B the reaction of an acid anhydride with ammonia O C. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Primary secondary or tertiary different amines can be used.
Amines are prepared by the following methods. Accordingly amides are prepared from reactive carboxylic acid derivatives esters anhydrides or acyl chlorides and amines. Extremity blocks for the arm may be established at any of the following nerves locations except.
Imidoyl chlorides can be obtained from a range of aliphatic and aromatic amides by a general synthetic method involving the reaction with phosphorus penta- and trihalides in a variety of solvents Equation 3. However there are some more methods. You may also like.
In an alternative method amides were formed in the reaction of amines 48 with trimethylaluminum and carboxylic acid esters in toluene or hexane Scheme 51. Aldehydes and ketones can be converted into 1 o 2 o and 3 o amines using reductive amination. Nitroalkanes can also be similarly reduced to the corresponding alkanamines.
Action of Ammonia on Alcohol. Wherein R 1 to R 4 are the same or. Chemistry Practice Paper for NEET.
Question 20 Amides may be prepared by which of the following methods. This method yields a mixture of 1 o 2 o 3 o amines and 4 o salts which are separated from each other by means of Hinsberg method Hofmann method and fractional distillation. The alkylation of ammonia Gabriel synthesis reduction of nitriles reduction of amides reduction of nitrocompounds and reductive amination of aldehydes and ketones are methods commonly used for preparing amines.
Esters or amides of trimellitc anhydride can be prepared by reacting the latter directly with either a polyol or a disecondary amine at elevated temperatures and by a rearrangement reaction. Reduction of nitro compounds Nitro compounds are reduced to amines by passing hydrogen gas in the presence of finely divided nickel palladium or platinum and also by reduction with metals in acidic medium. Amides are ordinarily prepared by a reaction of acid chlorides with ammonia or amines.
When hydrogen gas is passed over nitro compounds in the presence of finely divided nickel palladium or platinum these are reduced to corresponding amines. You can also react ammonia with esters to prepare. One of the simplest ways of preparing an amide is the treatment of an ester acyl chloride or anhydride.
However amines can be prepared in good yield by using excess of. The most convenient method to prepare primary i Amine amine containing one carbon atom less is a Gabriel phthalmidie synthesis b Reductive amination of aldehydes c Hofmann bromamide reaction d Reduction of isonitriles. By this method you are retaining the number of carbon atoms in both amide and the amine.
Aldehydes ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl group. The reaction of a carboxylic acid and a nitrate D the reaction of a carboxylic acid and an ammonium salt O E Both A and B are correct. The amide function formally arises from the condensation of a carboxylic acid with an amine.
An amide is prepared by reacting an acid halide with ammonia. Amides were prepared by treatment of 9-aminoalkaloids 48 with acid chlorides in DCM in the presence of Et 3 N as a base. Reduction of Nitro Compounds.
C Hofmann bromamide reaction. Amides can be prepared from an acid chloride and depending on the substitution pattern desired ie.

Amides Preparation And Reactions Summary Chemistry Steps
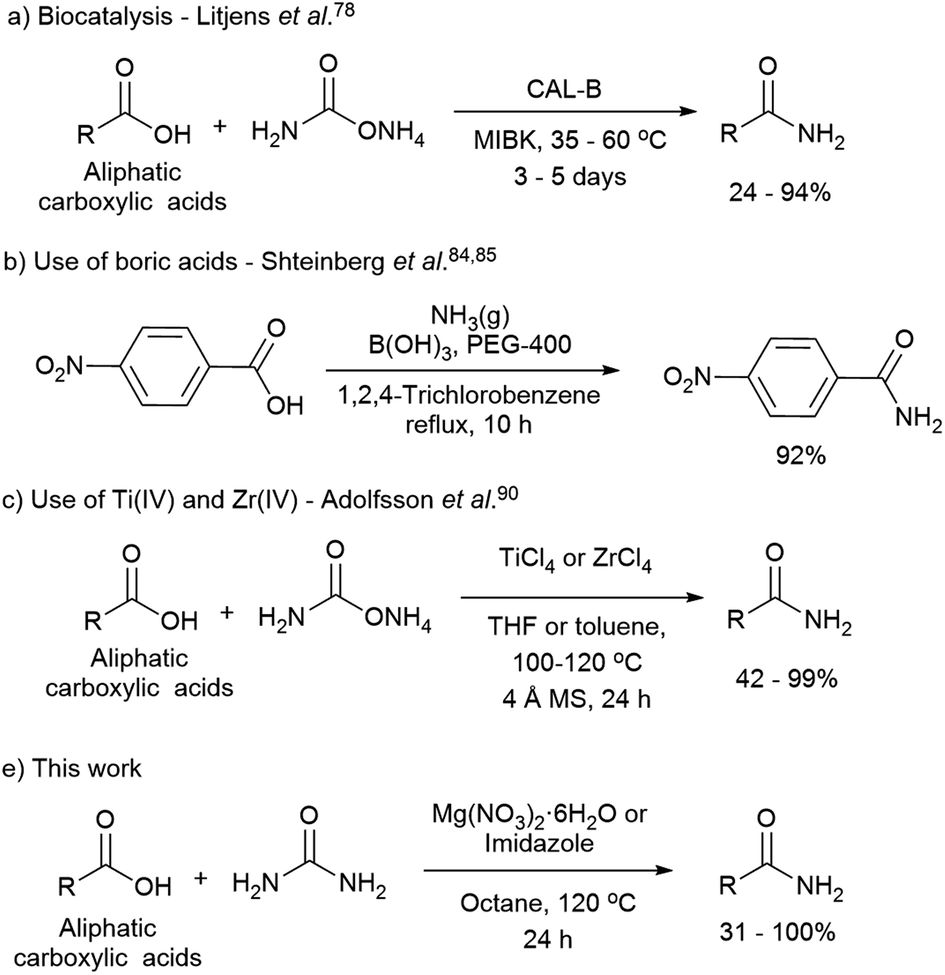
Direct Synthesis Of Amides From Nonactivated Carboxylic Acids Using Urea As Nitrogen Source And Mg No 3 2 Or Imidazole As Catalysts Chemical Science Rsc Publishing Doi 10 1039 D0sc01317j
Molecules Free Full Text Amide Activation In Ground And Excited States Html
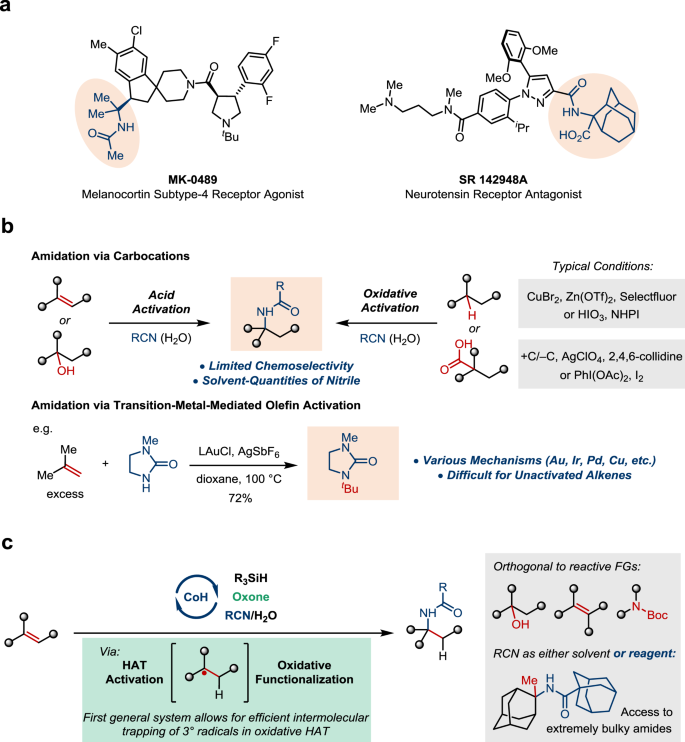
Highly Chemoselective Synthesis Of Hindered Amides Via Cobalt Catalyzed Intermolecular Oxidative Hydroamidation Nature Communications

A Summary Of The Kiliani Fischer Synthesis Organic Chemistry Study Chemistry Lessons Chemistry

Wittig Reaction Examples Wittig Reaction Chemistry Lessons Chemistry
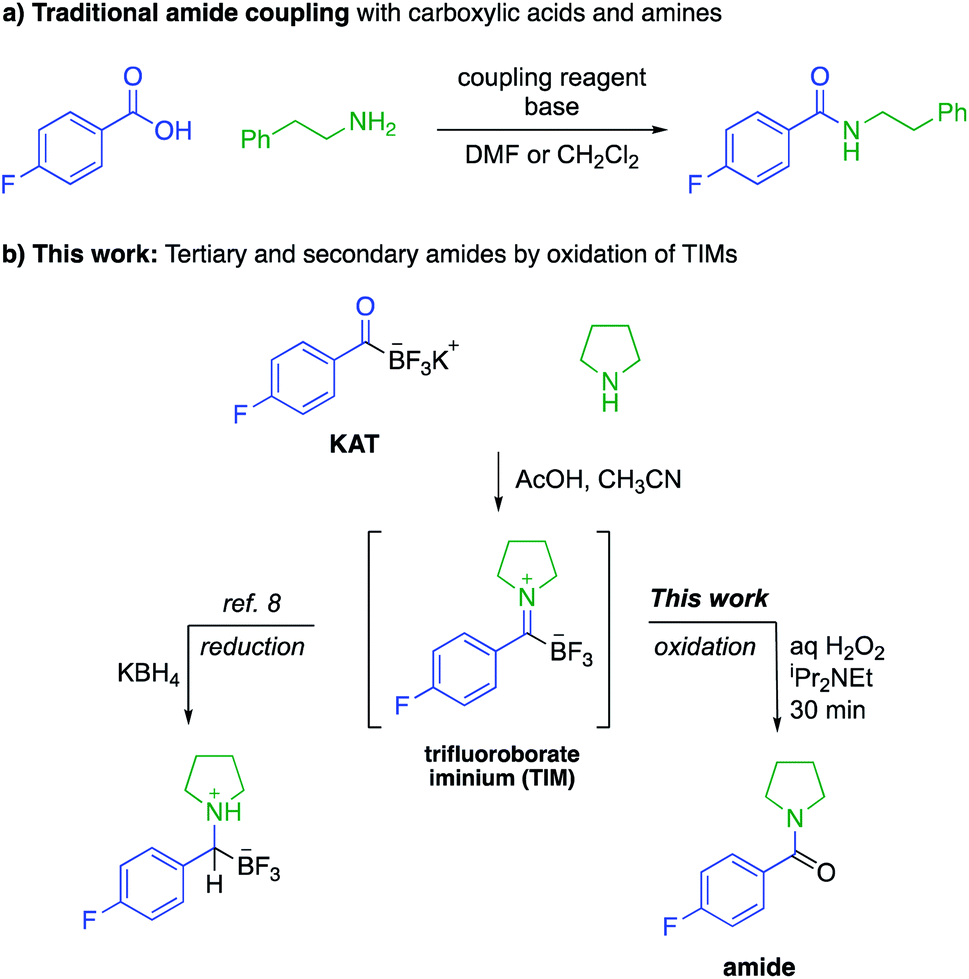
Synthesis Of Secondary And Tertiary Amides Without Coupling Agents From Amines And Potassium Acyltrifluoroborates Kats Chemical Science Rsc Publishing Doi 10 1039 D0sc01330g

Amides Preparation And Reactions Summary Chemistry Steps
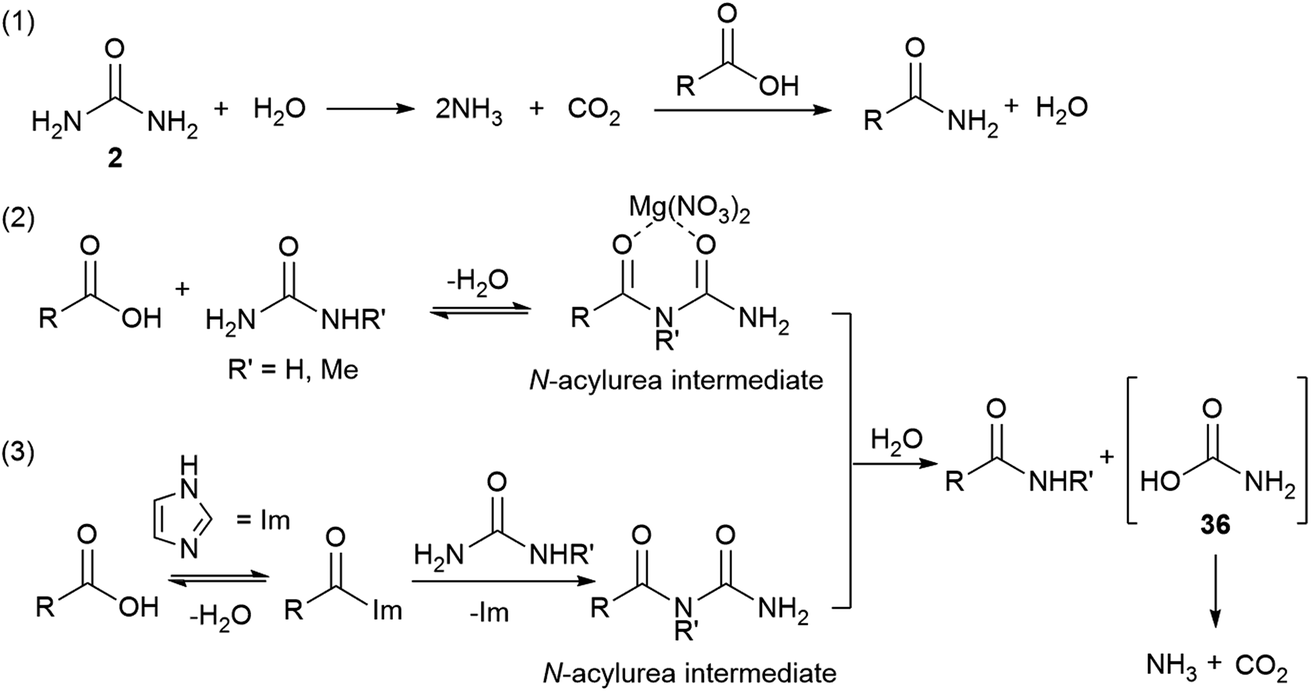
Direct Synthesis Of Amides From Nonactivated Carboxylic Acids Using Urea As Nitrogen Source And Mg No 3 2 Or Imidazole As Catalysts Chemical Science Rsc Publishing Doi 10 1039 D0sc01317j

Metal Catalyzed Dehydration Of Primary Amides To Nitriles Al Huniti 2019 Asian Journal Of Organic Chemistry Wiley Online Library

Amide Bond Formation Strategies Latest Advances On A Dateless Transformation Massolo 2020 European Journal Of Organic Chemistry Wiley Online Library

Pin On Nucleophilic Substitution Reaction Sn1 And Sn2
Organic Nitrogen Compounds Vii Amides The Rest Of The Story

Amides Preparation And Reactions Summary Chemistry Steps
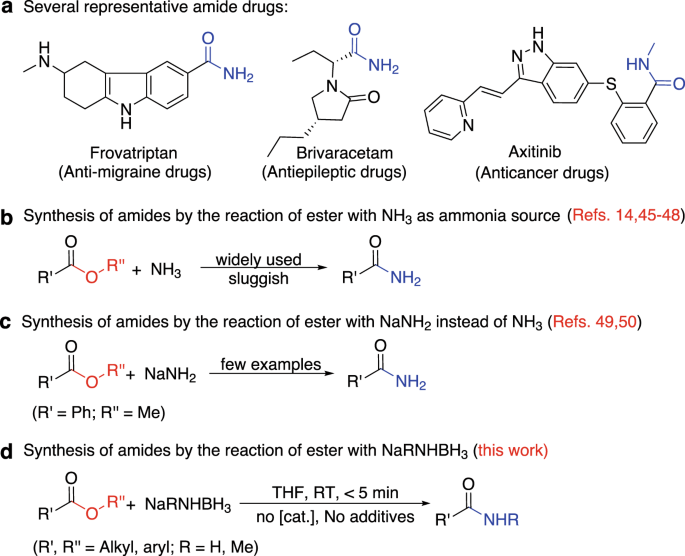
Efficient Synthesis Of Primary And Secondary Amides Via Reacting Esters With Alkali Metal Amidoboranes Nature Communications
Organic Nitrogen Compounds Vii Amides The Rest Of The Story

Preparation Of Some Homopolymers Polymer Good Notes Chemistry Notes


Comments
Post a Comment